Deshidroepiandrosterona: Diferenzas entre revisións
Nova páxina: "{{entradución}} thumb|right|Deshidroepiandrosterona.<br>[[Número CAS: 53-43-0. [[Ficheiro:Dehidroepiandrosterona3D.png|thumb|right|Modelo 3D da deshidroe..." |
(Sen diferenzas.)
|
Revisión como estaba o 3 de marzo de 2012 ás 11:51
Este artigo está a ser traducido ao galego por un usuario desta Wikipedia; por favor, non o edite. O usuario Miguelferig (conversa · contribucións) realizou a última edición na páxina hai 12 anos. Se o usuario non publica a tradución nun prazo de trinta días, procederase ó seu borrado rápido. |
[[Ficheiro:DHEA.png|thumb|right|Deshidroepiandrosterona.
Número CAS: 53-43-0.
[[Ficheiro:Dehidroepiandrosterona3D.png|thumb|right|Modelo 3D da deshidroepiandrosterona.
Nome IUPC:(3S,8R,9S,10R,13S,14S)-3-hidroxi-10,13-dimetil-1,2,3,4,7,8,9,11,12,14,15,16-dodecahidrociclopenta[a]fenantren-17-ona.]]
A 5-deshidroepiandrosterona (5-DHEA) é unha hormona esteroide endóxena de 19 carbonos [1]. É o principal produto esteroide producido polas glándulas adrenais[2] e tamén se producen nas gónadas e o cerebro, e a partir del se sintetizan outros esteroides.[3] A DHEA é o esteroide circulante máis abundante nos humanos.[4]
DHEA has been implicated in a broad range of biological effects in humans and other mammals. It acts on the androgen receptor both directly and through its metabolites, which include androstenediol and androstenedione, which can undergo further conversion to produce the androgen testosterone and the estrogens, including estrone, estradiol, and estriol.[5] DHEA is also a potent sigma-1 agonist.[6] It is considered a neurosteroid.[5]
Effects and uses
In women with adrenal insufficiency and the healthy elderly there is insufficient evidence to support the use of DHEA.[7][8]
Strength
Evidence is inconclusive in regards to the effect of DHEA on strength in the elderly.[9]
In younger men, no statistically significant effect of DHEA supplementation on lean body mass, strength, or testosterone levels was found in a randomized placebo-controlled trial.[10]
Memory
DHEA supplementation has not been found to be useful for memory function in normal middle aged or older adults.[11] It has been studied as a treatment for Alzheimer's disease, but there is no evidence that it is effective.[12]
Female reproductive health
Since 2000, DHEA supplementation has been used in reproductive medicine in combination with gonadotropins as a way to treat female infertility.[13]
Cardiovascular disease and risk of death
A review in 2003 found the then-extant evidence sufficient to suggest that low serum levels of DHEAS may be associated with coronary heart disease in men, but insufficient to determine whether DHEA supplementation would have any cardiovascular benefit.[14]
A 1986 study found that a higher level of endogenous DHEA, as determined by a single measurement, correlated with a lower risk of death or cardiovascular disease.[15] However, a more recent 2006 study found no correlation between DHEA levels and risk of cardiovascular disease or death in men.[16] A 2007 study found the DHEA restored oxidative balance in diabetic patients, reducing tissue levels of pentosidine—a biomarker for advanced glycation endproducts.[17]
Lupus
There is some evidence of short term benefit in those with systemic lupus erythematosus but little evidence of long term benefit or safety.[18]
Safety
5-DHEA is produced naturally in the human body and has a safe history of use for the past 15 years. In a study by Chang et al, 3b-hydroxy-androst-5-ene-17-one (5-DHEA) was administered at a dose of 200 mg/day for 24 weeks with only slight androgenic effects noted [19]. Another study utilized a dose up to 400 mg/day for 8 weeks with few adverse events reported [20] . A longer term study followed patients dosed with 50 mg of 3b-hydroxy-androst-5-ene-17-one for 12 months with the number and severity of side effects reported to be small.[21] Another study delivered a dose of 50 mg of 3b-hydroxy-androst-5-ene-17-one for 10 months with no serious adverse events reported. [22] A multitude of additional studies utilizing doses from 25 mg up to 2,250 mg per day for up to 24 months of therapy in men, women and even children have demonstrated that 3b-hydroxy-androst-5-ene-17-one is well-tolerated and has numerous benefits with no serious adverse events reported.
As a hormone precursor, there has been a smattering of reports of side effects possibly caused by the hormone metabolites of DHEA.[23][24]
Dehydroepiandrosterone sulfate
Dehydroepiandrosterone sulfate (DHEAS) is the sulfated version of DHEA. This conversion is reversibly catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, and small intestine. In the blood, most DHEA is found as DHEAS with levels that are about 300 times higher than those of free DHEA. Orally ingested DHEA is converted to its sulfate when passing through intestines and liver. Whereas DHEA levels naturally reach their peak in the early morning hours, DHEAS levels show no diurnal variation. From a practical point of view, measurement of DHEAS is preferable to DHEA, as levels are more stable.[Cómpre referencia]
Production
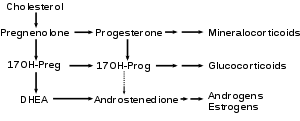
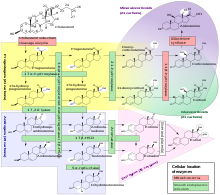
DHEA is produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage); then another enzyme, CYP17A1, converts pregnenolone to 17α-Hydroxypregnenolone and then to DHEA.[25]
Mechanism of action
DHEA can be understood as a prohormone for the sex steroids. DHEAS may be viewed as buffer and reservoir. As most DHEA is produced by the zona reticularis of the adrenal cortex, it is argued that there is a role in the immune and stress response.Modelo:Who
As almost all DHEA is derived from the adrenal glands, blood measurements of DHEAS/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have elevated levels of DHEAS.[26]
Increasing endogenous production
Regular exercise is known to increase DHEA production in the body.[27][28] Calorie restriction has also been shown to increase DHEA in primates.[29] Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction.[30]
Isomers
The term "dehydroepiandrosterone" is ambiguous chemically because it does not include the specific positions within epiandrosterone at which hydrogen atoms are missing. DHEA has a number of naturally occurring isomers that may have similar pharmacological effects. Some isomers of DHEA are 1-dehydroepiandrosterone and 4-dehydroepiandrosterone. These isomers are also technically DHEA, since they are dehydroepiandrosterones in which hydrogens are removed from the epiandrosterone skeleton.
Society and culture
Legality
United States
DHEA is legal to sell in the United States as a dietary supplement. It is currently grandfathered in as an "Old Dietary Ingredient" being on sale prior to 1994. DHEA is specifically exempted from the Anabolic Steroid Control Act of 1990 and 2004 [31]
Canada
In Canada, a prescription is required to buy DHEA.[32]
Sports and athletics
DHEA is a prohibited substance under the World Anti-Doping Code of the World Anti-Doping Agency,[33] which manages drug testing for Olympics and other sports. In January 2011, NBA player O.J. Mayo was given a 10-game suspension after testing positive for DHEA. Mayo termed his use of DHEA as "an honest mistake".[34] Mayo is the seventh player to test positive for performance-enhancing drugs since the league began testing in 1999. Rashard Lewis, then with the Orlando Magic, tested positive for DHEA and was suspended 10 games before the start of the 2009-10 season.[35]
Synonyms and brand names
Synonyms for dehydroepiandrosterone:
The International Nonproprietary Name is "prasterone". Systematic names include 3-beta-hydroxy-5-androsten-17-one, 3-beta-hydroxyandrost-5-en-17-one, 3beta-hydroxy-5-androsten-17-one, 3beta-hydroxy-androst-5-en-17-one, 3beta-hydroxy-D5-androsten-17-one, 3beta-hydroxyandrost-5-en-17-one, 3beta-hydroxyandrost-5-ene-17-one, 3-beta-hydroxy-etioallocholan-5-ene-17-one, and 5-androsten-3beta-ol-17-one. Fidelin is Paladin's trade name for DHEA.
Fluasterone is a synthetic derivative of DHEA.
Marketing
In the United States, DHEA or DHEAS have been advertised with claims that they may be beneficial for a wide variety of ailments. DHEA and DHEAS are readily available in the United States, where they are marketed as over-the-counter dietary supplements.[36] A 2004 review in the American Journal of Sports Medicine concluded that "The marketing of this supplement's effectiveness far exceeds its science."[37]
Investigación
Cáncer
Some in vitro studies have found DHEA to have both antiproliferative and yet also apoptotic effect on cancer cell lines.[38][39][40] The clinical significance of these findings, if any, is unknown. Higher levels of DHEA and other endogenous sex hormones are strongly associated with an increased risk of developing breast cancer in both pre- and postmenopausal women.[41][42]
Notas
- ↑ The NIH National Library of Medicine — Dehydroepiandrosterone http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-dhea.html
- ↑ The Merck Index, 13th Edition, 7798
- ↑
Schulman, Robert A., M.D.; Dean, Carolyn, M.D. (2007). Solve It With Supplements. New York City: Rodale, Inc. p. 100. ISBN 978-1-57954-942-8.
DHEA (Dehydroepiandrosterone) is a common hormone produced in the adrenal glands, the gonads, and the brain.
A referencia usa o parámetro obsoleto|coauthors=(Axuda) - ↑ William F Ganong MD, 'Review of Medical Physiology', 22nd Ed, McGraw Hill, 2005, page 362.
- ↑ 5,0 5,1 Mo Q, Lu SF, Simon NG (2006). "Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity". J. Steroid Biochem. Mol. Biol. 99 (1): 50–8. PMID 16524719. doi:10.1016/j.jsbmb.2005.11.011. Parámetro descoñecido
|month=ignorado (Axuda) - ↑ Romieu, P.; Martin-Fardon, R.; Bowen, W. D.; and Maurice, T. (2003). Sigma 1 Receptor-Related Neuroactive Steroids Modulate Cocaine-Induced Reward. 23(9): 3572.
- ↑ Arlt, W (2004 Sep). "Dehydroepiandrosterone and ageing.". Best practice & research. Clinical endocrinology & metabolism 18 (3): 363–80. PMID 15261843.
- ↑ Alkatib, AA; Cosma, M, Elamin, MB, Erickson, D, Swiglo, BA, Erwin, PJ, Montori, VM (2009 Oct). "A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency.". The Journal of clinical endocrinology and metabolism 94 (10): 3676–81. PMID 19773400. A referencia usa o parámetro obsoleto
|coauthors=(Axuda) - ↑ Baker, WL; Karan, S, Kenny, AM (2011 Jun). "Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review.". Journal of the American Geriatrics Society 59 (6): 997–1002. PMID 21649617. A referencia usa o parámetro obsoleto
|coauthors=(Axuda) - ↑ Wallace, M. B.; Lim, J.; Cutler, A.; Bucci, L. (1999). "Effects of dehydroepiandrosterone vs androstenedione supplementation in men". Medicine and Science in Sports and Exercise 31 (12): 1788–92. PMID 10613429. doi:10.1097/00005768-199912000-00014.
- ↑ Grimley Evans, J; Malouf, R, Huppert, F, van Niekerk, JK (2006 Oct 18). "Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people.". Cochrane database of systematic reviews (Online) (4): CD006221. PMID 17054283. A referencia usa o parámetro obsoleto
|coauthors=(Axuda) - ↑ Fuller, SJ; Tan, RS, Martins, RN (2007 Sep). "Androgens in the etiology of Alzheimer's disease in aging men and possible therapeutic interventions.". Journal of Alzheimer's disease : JAD 12 (2): 129–42. PMID 17917157. A referencia usa o parámetro obsoleto
|coauthors=(Axuda) - ↑ Casson PR, et al. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod, 2000;15:2129-2132.
- ↑ Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol. 2003 Oct;58(5):403-10. PMID 14609305
- ↑ Barrett-Connor, E.; Khaw, K. T.; Yen, S. S. (1986). "A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease". N. Engl. J. Med. 315 (24): 1519–24. PMID 2946952. doi:10.1056/NEJM198612113152405.
- ↑ Arnlöv, J.; Pencina, M. J.; Amin, S.; et al. (2006). "Endogenous sex hormones and cardiovascular disease incidence in men". Ann. Intern. Med. 145 (3): 176–84. PMID 16880459.
- ↑ Boggs, Will. "DHEA Restores Oxidative Balance in Type 2 Diabetes". Medscape. Arquivado dende o orixinal o 2008-01-07. Consultado o 2007-12-14.
- ↑ Crosbie, D; Black, C, McIntyre, L, Royle, PL, Thomas, S (2007 Oct 17). "Dehydroepiandrosterone for systemic lupus erythematosus.". Cochrane database of systematic reviews (Online) (4): CD005114. PMID 17943841. A referencia usa o parámetro obsoleto
|coauthors=(Axuda) - ↑ Chang DM, Lan JL, Lin HY, Luo SF. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7.
- ↑ Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006 Jan;163(1):59-66.
- ↑ Brooke AM, Kalingag LA, Miraki-Moud F, Camacho-Hübner C, Maher KT, Walker DM, Hinson JP, Monson JP. Dehydroepiandrosterone improves psychological well-being in male and female hypopituitary patients on maintenance growth hormone replacement. J Clin Endocrinol Metab. 2006 Oct;91(10):3773-9. Epub 2006 Jul 18.
- ↑ Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E1003-8.
- ↑ Medline Plus. "DHEA". Drugs and Supplements Information. National Library of Medicine. Consultado o 18 February 2010.
- ↑ Medscape (2010). "DHEA Oral". Drug Reference. WebMD LLC. Consultado o 18 February 2010.
- ↑ Harper's illustrated Biochemistry, 27th edition, Ch.41 "The Diversity of the Endocrine system"
- ↑ Roczniki Akademii Medycznej w Białymstoku · Vol. 48, 2003 Annales Academiae Medicae Bialostocensis Incidence of elevated LH/FSH ratioin polycystic ovary syndrome women with normo- and hyperinsulinemiaBanaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L
- ↑ Eur. J. Appl. Physiol. Occup. Physiol. 1998 Oct; 78(5):466-71
- ↑ J. Gerontol. A. Biol. Sci. Med. Sci. 2002 Apr; 57(4):B158-65
- ↑ Mattison, Julie A.; Lane, Mark A.; Roth, George S.; Ingram, Donald K. (2003). "Calorie restriction in rhesus monkeys". Experimental Gerontology 38 (1–2): 35–46. PMID 12543259. doi:10.1016/S0531-5565(02)00146-8..
- ↑ Roberts, E. (1999). "The importance of dehydroepiandrosterone sulfate in the blood of primates: a longer and healthier life?". Biochemical Pharmacology 57 (4): 329–346. PMID 9933021. doi:10.1016/S0006-2952(98)00246-9..
- ↑ http://www.deadiversion.usdoj.gov/fed_regs/rules/2005/fr1216.htm
- ↑ Dr. Michael Colgin. The Deal With D.H.E.A. Vista Magazine Online. www.vistamag.com [1]
- ↑ World Anti-Doping Agency
- ↑ Memphis Grizzlies' O.J. Mayo gets 10-game drug suspension - ESPN. January 27, 2011.
- ↑ [2]
- ↑ Calfee, R.; Fadale, P. (2006). "Popular ergogenic drugs and supplements in young athletes". Pediatrics 117 (3): e577–89. PMID 16510635. doi:10.1542/peds.2005-1429.
In 2004, a new Steroid Control Act that placed androstenedione under Schedule III of controlled substances effective January 2005 was signed. DHEA was not included in this act and remains an over-the-counter nutritional supplement.
Parámetro descoñecido|month=ignorado (Axuda) - ↑ Tokish, J. M.; Kocher, M. S.; Hawkins, R. J. (2004). "Ergogenic aids: a review of basic science, performance, side effects, and status in sports". The American Journal of Sports Medicine 32 (6): 1543–53. PMID 15310585. doi:10.1177/0363546504268041.
- ↑ Yang, N. C.; Jeng, K. C.; Ho, W. M.; Hu, M. L. (2002). "ATP depletion is an important factor in DHEA-induced growth inhibition and apoptosis in BV-2 cells". Life Sci. 70 (17): 1979–88. PMID 12148690. doi:10.1016/S0024-3205(01)01542-9.
- ↑ Schulz, S.; Klann, R. C.; Schönfeld, S.; Nyce, J. W. (1992). "Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: role of isoprenoid biosynthesis". Cancer Res. 52 (5): 1372–6. PMID 1531325.
- ↑ Loria, R. M. (2002). "Immune up-regulation and tumor apoptosis by androstene steroids". Steroids 67 (12): 953–66. PMID 12398992. doi:10.1016/S0039-128X(02)00043-0.
- ↑ Tworoger, S. S.; Missmer, S. A.; Eliassen, A. H.; et al. (2006). "The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women". Cancer Epidemiol. Biomarkers Prev. 15 (5): 967–71. PMID 16702378. doi:10.1158/1055-9965.EPI-05-0976.
- ↑ Key, T.; Appleby, P.; Barnes, I.; Reeves, G. (2002). "Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies". J. Natl. Cancer Inst. 94 (8): 606–16. PMID 11959894.
Ligazóns externas
- Information on DHEA from the Mayo Clinic
- DHEA in elderly women and DHEA or testosterone in elderly men, published in the New England Journal of Medicine in 2006. "Neither DHEA nor low-dose testosterone replacement in elderly people has physiologically relevant beneficial effects on body composition, physical performance, insulin sensitivity, or quality of life."
- DHEA, from the Skeptic's Dictionary
- ChemSub Online: Dehydroepiandrosterone - DHEA
