Flavona

As flavonas (do latín flavus, 'amarelo'), son unha clase de flavonoides baseada nun esqueleto de 2-fenilcromen-4-ona (2-fenil-1-benzopiran-4-ona) (imaxe da dereita).[1][2]
As flavonas son comúns nos alimentos, principalmente nas especias, e froitos e vexetais vermellos e púrpuras.[1] Entre as flavonas comúns están a apixenina (4',5,7-trihidroxiflavona), luteolina (3',4',5,7-tetrahidroxiflavona), tanxeritina (4',5,6,7,8-pentametoxiflavona), crisina (5,7-dihidroxiflavona) e 6-hidroxiflavona.[1]
Inxestión e eliminación[editar | editar a fonte]
As flavonas encóntranse principalmente en especias e plantas vermellas e púrpuras.[1] O consumo diario estimado de flavonas é duns 2 mg.[1] As flavonas non presentan efectos fisiolóxicos no corpo humano e non teñen ningún poder como antioxidantes.[1][3] Despois da súa inxestión e metabolización, as flavonas, outros polifenois e os seus metabolitos son absorbidos bastante pouco polos órganos do corpo e son excretados rapidamente pola urina, o que indica a súa presumida ausencia de papeis no metabolismo do corpo.[1][4]
Interaccións con fármacos[editar | editar a fonte]
As flavonas teñen efectos sobre a actividade do CYP (P450)[5][6] que son encimas que metabolizan a maioría dos fármacos do corpo.
Química orgánica[editar | editar a fonte]
En química orgánica hai varios métodos para a síntese de flavonas:
- Reacción de Allan–Robinson
- Síntese de Auwers
- Rearranxo de Baker–Venkataraman
- Reacción de Algar–Flynn–Oyamada
Outros métodos son a ciclación deshidratante de certas 1,3-diaril dicetonas.[7]

Rearranxo de Wessely–Moser[editar | editar a fonte]
O rearranxo ou redistribución de Wessely–Moser (1930)[8] foi unha feramenta importante para a dilucidación da estrutura dos flavonoides. Implica a conversión da 5,7,8-trimetoxiflavona en 5,6,7-trihidroxiflavona con hidrólise dos grupos metoxi a grupos fenol. Tamén ten un potencial sintético, por exemplo:[9]
Esta reacción de redistribución ten lugar en varios pasos: A apertura do anel á dicetona, B rotación do enlace con formación dunha interacción favorable con fenil-cetona tipo acetilcetona, e C hidrólise de dous grupos metoxi e peche do anel.
Flavonas comúns[editar | editar a fonte]
| Nome | Estrutura | R3 | R5 | R6 | R7 | R8 | R2' | R3' | R4' | R5' | R6' |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esqueleto de flavona | 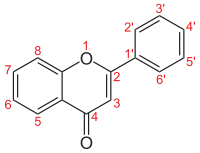
|
– | – | – | – | – | – | – | – | – | – |
| Primuletina | – | –OH | – | – | – | – | – | – | – | – | |
| Crisina | – | –OH | – | –OH | – | – | – | – | – | – | |
| Tectocrisina | – | –OH | – | –OCH3 | – | – | – | – | – | – | |
| Primetina | – | –OH | – | – | –OH | – | – | – | – | – | |
| Apixenina | – | –OH | – | –OH | – | – | – | –OH | – | – | |
| Acacetina | – | –OH | – | –OH | – | – | – | –OCH3 | – | – | |
| Genkwanina | – | –OH | – | –OCH3 | – | – | – | –OH | – | – | |
| Equinoidina | – | –OH | – | –OCH3 | – | –OH | – | – | – | – | |
| Baicaleína | – | –OH | –OH | –OH | – | – | – | – | – | – | |
| Oroxilon | – | –OH | –OCH3 | –OH | – | – | – | – | – | – | |
| Negleteína | – | –OH | –OH | –OCH3 | – | – | – | – | – | – | |
| Norwogonina | – | –OH | – | –OH | –OH | – | – | – | – | – | |
| Wogonina | – | –OH | – | –OH | –OCH3 | – | – | – | – | – | |
| Geraldona | – | – | – | –OH | – | – | –OCH3 | –OH | – | – | |
| Titonina | – | – | – | –OCH3 | – | – | –OH | –OCH3 | – | – | |
| Luteolina | – | –OH | – | –OH | – | – | –OH | –OH | – | – | |
| 6-Hidroxiluteolina | – | –OH | –OH | –OH | – | – | –OH | –OH | – | – | |
| Crisoeriol | – | –OH | – | –OH | – | – | –OCH3 | –OH | – | – | |
| Diosmetina | – | –OH | – | –OH | – | – | –OH | –OCH3 | – | – | |
| Pilloína | – | –OH | – | –OCH3 | – | – | –OH | –OCH3 | – | – | |
| Velutina | – | –OH | – | –OCH3 | – | – | –OCH3 | –OH | – | – | |
| Norartocarpetina | – | –OH | – | –OH | – | –OH | – | –OH | – | – | |
| Artocarpetina | – | –OH | – | –OCH3 | – | –OH | – | –OH | – | – | |
| Escutelareína | – | –OH | –OH | –OH | – | – | – | –OH | – | – | |
| Hispidulina | – | –OH | –OCH3 | –OH | – | – | – | –OH | – | – | |
| Sorbifolina | – | –OH | –OH | –OCH3 | – | – | – | –OH | – | – | |
| Pectolinarixenina | – | –OH | –OCH3 | –OH | – | – | – | –OCH3 | – | – | |
| Cirsimaritina | – | –OH | –OCH3 | –OCH3 | – | – | – | –OH | – | – | |
| Mikanin | – | –OH | –OCH3 | –OCH3 | – | – | – | –OCH3 | – | – | |
| Isoscutelareína | – | –OH | – | –OH | –OH | – | – | –OH | – | – | |
| Zapotinina | – | –OH | –OCH3 | – | – | –OCH3 | – | – | – | –OCH3 | |
| Zapotina | – | –OCH3 | –OCH3 | – | – | –OCH3 | – | – | – | –OCH3 | |
| Cerrosilina | – | –OCH3 | –OCH3 | – | – | – | –OCH3 | – | –OCH3 | – | |
| Alnetina | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | – | – | – | – | |
| Tricetina | – | –OH | – | –OH | – | – | –OH | –OH | –OH | – | |
| Tricina | – | –OH | – | –OH | – | – | –OCH3 | –OH | –OCH3 | – | |
| Corimbosina | – | –OH | – | –OCH3 | – | – | –OCH3 | –OCH3 | –OCH3 | – | |
| Nepetina | – | –OH | –OCH3 | –OH | – | – | –OH | –OH | – | – | |
| Pedalitina | – | –OH | –OH | –OCH3 | – | – | –OH | –OH | – | – | |
| Nodifloretina | – | –OH | –OH | –OH | – | – | –OCH3 | –OH | – | – | |
| Jaceosidina | – | –OH | –OCH3 | –OH | – | – | –OCH3 | –OH | – | – | |
| Cirsiliol | – | –OH | –OCH3 | –OCH3 | – | – | –OH | –OH | – | – | |
| Eupatilina | – | –OH | –OCH3 | –OH | – | – | –OCH3 | –OCH3 | – | – | |
| Cirsilineol | – | –OH | –OCH3 | –OCH3 | – | – | –OCH3 | –OH | – | – | |
| Eupatorina | – | –OH | –OCH3 | –OCH3 | – | – | – | –OCH3 | –OH | – | |
| Sinensetina | – | –OCH3 | –OCH3 | –OCH3 | – | – | – | –OCH3 | –OCH3 | – | |
| Hipolaetina | – | –OH | – | –OH | –OH | – | –OH | –OH | – | – | |
| Onopordina | – | –OH | – | –OH | –OCH3 | – | –OH | –OH | – | – | |
| Wightina | – | –OH | – | –OCH3 | –OCH3 | –OCH3 | –OH | – | – | – | |
| Nevadensina | – | –OH | –OCH3 | –OH | –OCH3 | – | – | –OCH3 | – | – | |
| Xantomicrol | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | – | –OH | – | – | |
| Tanxeretina | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | – | –OCH3 | – | – | |
| Serpilina | – | –OH | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | – | |
| Sudaquitina | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OH | – | – | |
| Acerosina | – | –OH | –OCH3 | –OH | –OCH3 | – | –OH | –OCH3 | – | – | |
| Himenoxina | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OCH3 | – | – | |
| Gardenina D | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | –OH | –OCH3 | – | – | |
| Nobiletina | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | –OCH3 | –OCH3 | – | – | |
| Escaposina | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OCH3 | –OH | ||
| Nome | Estrutura | R3 | R5 | R6 | R7 | R8 | R2' | R3' | R4' | R5' | R6' |
Notas[editar | editar a fonte]
- ↑ 1,0 1,1 1,2 1,3 1,4 1,5 1,6 "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. November 2015. Consultado o 30 March 2018.
- ↑ "Flavone". ChemSpider, Royal Society of Chemistry. 2015. Consultado o 30 March 2018.
- ↑ Lotito, S; Frei, B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon?". Free Radical Biology and Medicine 41 (12): 1727–46. PMID 17157175. doi:10.1016/j.freeradbiomed.2006.04.033.
- ↑ David Stauth (5 March 2007). "Studies force new view on biology of flavonoids". EurekAlert!; Adaptado dunhas noticias da Universidde do estado de Oregón.
- ↑ Cermak R, Wolffram S., The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms,Curr Drug Metab. 2006 Oct;7(7):729-44.
- ↑ Si D, Wang Y, Zhou YH, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metab. Dispos. 37 (3): 629–34. PMID 19074529. doi:10.1124/dmd.108.023416. Arquivado dende o orixinal o 29 de maio de 2020. Consultado o 23 de novembro de 2018.[1] Arquivado 17 de decembro de 2008 en Wayback Machine.
- ↑ Sarda SR, Pathan MY, Paike VV, Pachmase PR, Jadhav WN, Pawar RP (2006). "A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation". Arkivoc xvi: 43–8. doi:10.3998/ark.5550190.0007.g05.
- ↑ Wessely F, Moser GH (December 1930). "Synthese und Konstitution des Skutellareins". Monatsh. Chem. 56 (1): 97–105. doi:10.1007/BF02716040. (require subscrición (?)).
- ↑ Larget R, Lockhart B, Renard P, Largeron M (April 2000). "A convenient extension of the Wessely-Moser rearrangement for the synthesis of substituted alkylaminoflavones as neuroprotective agents in vitro". Bioorg. Med. Chem. Lett. 10 (8): 835–8. PMID 10782697. doi:10.1016/S0960-894X(00)00110-4.
- ↑ The Flavonoids - Springer. doi:10.1007/978-1-4899-2909-9.
Véxase tamén[editar | editar a fonte]
Ligazóns externas[editar | editar a fonte]
- Flavones Medical Subject Headings (MeSH) na Biblioteca Nacional de Medicina dos EUA.

