Luteína: Diferenzas entre revisións
Sen resumo de edición |
Sen resumo de edición |
||
| Liña 78: | Liña 78: | ||
A luteína obtéñena os animais directa ou indirectamente das plantas,e pode ter funcións antioxidantes e absorbe luz azul. A luteína atópase na xema dos ovos e nas graxas animais. Nos polos, ademais de dar cor ás xemas dos ovos, dálle cor amarela á pel e graxas, e utilízase na alimentación dos polos para ese propósito. A [[retina]] humana acumula luteína e [[zeaxantina]]. Esta última predomina na [[mácula da retina|mácula lútea]], mentres que a luteína predomina no resto da retina. Alí pode ter funcións fotoprotectoras para a retina evitando os danos producidos polos [[radical libre|radicais libres]] producidos pola luz azul. Tamén se encontra no [[corpo lúteo]] dos [[ovario]]s dos mamíferos. |
A luteína obtéñena os animais directa ou indirectamente das plantas,e pode ter funcións antioxidantes e absorbe luz azul. A luteína atópase na xema dos ovos e nas graxas animais. Nos polos, ademais de dar cor ás xemas dos ovos, dálle cor amarela á pel e graxas, e utilízase na alimentación dos polos para ese propósito. A [[retina]] humana acumula luteína e [[zeaxantina]]. Esta última predomina na [[mácula da retina|mácula lútea]], mentres que a luteína predomina no resto da retina. Alí pode ter funcións fotoprotectoras para a retina evitando os danos producidos polos [[radical libre|radicais libres]] producidos pola luz azul. Tamén se encontra no [[corpo lúteo]] dos [[ovario]]s dos mamíferos. |
||
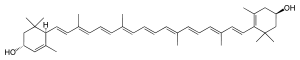
O principal [[estereoisómero]] natural da luteína é o (3''R'',3′''R'',6′''R'') |
O principal [[estereoisómero]] natural da luteína é o (3''R'',3′''R'',6′''R'')-''beta'',''epsilon''-caroteno-3,3′-diol. |
||
A luteína é unha molécula [[lipofílico|lipofílica]] e é case insoluble en auga. A presenza na molécula dunha longa rexión [[cromóforo|cromófora]] con dobres enlaces conxugados (cadea de [[polieno]]) dálle a súa capacidade de absorber luz. A cadea de polieno é susceptible de degradación oxidativa pola luz ou calor e é quimicamente inestable aos ácidos. |
A luteína é unha molécula [[lipofílico|lipofílica]] e é case insoluble en auga. A presenza na molécula dunha longa rexión [[cromóforo|cromófora]] con dobres enlaces conxugados (cadea de [[polieno]]) dálle a súa capacidade de absorber luz. A cadea de polieno é susceptible de degradación oxidativa pola luz ou calor e é quimicamente inestable aos ácidos. |
||
Revisión como estaba o 17 de xaneiro de 2015 ás 11:24
Este artigo está a ser traducido ao galego por un usuario desta Wikipedia; por favor, non o edite. O usuario Miguelferig (conversa · contribucións) realizou a última edición na páxina hai 9 anos. Se o usuario non publica a tradución nun prazo de trinta días, procederase ó seu borrado rápido. |
| Luteína | |
|---|---|
 | |
 | |
β,ε-caroteno-3,3'-diol | |
Outros nomes Luteína; trans-luteína; 4-[18-(4-Hydroxi-2,6,6-trimetil-1-ciclohexenil)-3,7,12,16-tetrametiloctadeca-1,3,5,7,9,11,13,15,17-nonaenil]-3,5,5-trimetil-ciclohex-2-en-1-ol | |
| Identificadores | |
| Número CAS | 127-40-2 |
| PubChem | 5281243 |
| ChemSpider | 4444655 |
| UNII | X72A60C9MT |
| ChEBI | CHEBI:28838 |
| ChEMBL | CHEMBL173929 |
| Imaxes 3D Jmol | Image 1 |
| |
| |
| Propiedades | |
| Fórmula molecular | C40H56O2 |
| Masa molar | 568,87 g mol−1 |
| Aspecto | sólido cristalino alaranxado-vermello |
| Punto de fusión | 190 °C; 374 °F; 463 K |
| Solubilidade en auga | Insoluble |
| Solubilidade en graxas | Soluble |
Se non se indica outra cousa, os datos están tomados en condicións estándar de 25 °C e 100 kPa. | |
A luteína (do latín luteus, "amarelo") é un carotenoide natural do grupo das xantofilas. A luteína sintetízana só as plantas e como outras xantofilas encóntrase en grandes cantidades en verduras e hortalizas como espinacas, col rizada e cenorias amarelas. Nas plantas verdes as xantofilas actúan modulando a enerxía da luz e serven como desexcitadores non fotoquímicos da clorofila triplete (unha forma excitada da clorofila), que se produce en exceso a altos niveis de luz durante a fotosíntese (ver ciclo das xantofilas).
A luteína obtéñena os animais directa ou indirectamente das plantas,e pode ter funcións antioxidantes e absorbe luz azul. A luteína atópase na xema dos ovos e nas graxas animais. Nos polos, ademais de dar cor ás xemas dos ovos, dálle cor amarela á pel e graxas, e utilízase na alimentación dos polos para ese propósito. A retina humana acumula luteína e zeaxantina. Esta última predomina na mácula lútea, mentres que a luteína predomina no resto da retina. Alí pode ter funcións fotoprotectoras para a retina evitando os danos producidos polos radicais libres producidos pola luz azul. Tamén se encontra no corpo lúteo dos ovarios dos mamíferos.
O principal estereoisómero natural da luteína é o (3R,3′R,6′R)-beta,epsilon-caroteno-3,3′-diol. A luteína é unha molécula lipofílica e é case insoluble en auga. A presenza na molécula dunha longa rexión cromófora con dobres enlaces conxugados (cadea de polieno) dálle a súa capacidade de absorber luz. A cadea de polieno é susceptible de degradación oxidativa pola luz ou calor e é quimicamente inestable aos ácidos.
A luteína está presente nas plantas en forma de éster de ácidos graxos, xa que se une a un ou dous ácidos graxos polos seus grupos hidroxilo. Por esta razón, a saponificación (des-esterficación) dos ésteres da luteína para liberar a luteína pode producir luteína nunha proporción molar de 1:1 a 1:2 con respecto aos ácidos graxos saponificados.
A luteína é un isómero da zeaxantina, da que se diferencia só pola situación dun dos dobres enlaces.
Como pigmento
This xanthophyll, like its sister compound zeaxanthin, has primarily been used as a natural colorant due to its orange-red color. Lutein absorbs blue light and therefore appears yellow at low concentrations and orange-red at high concentrations. Lutein is also anti angiogenic. It inhibits VEGF.
Lutein was traditionally used in chicken feed to improve the color of broiler chicken skin. Polled consumers viewed yellow chicken skin more favorably than white chicken skin. Such lutein fortification also results in a darker yellow egg yolk. Today the coloring of the egg yolk has become the primary reason for feed fortification. Lutein is not used as a colorant in other foods due to its limited stability, especially in the presence of other dyes.
Función no ollo humano
Lutein was found to be concentrated in the macula, a small area of the retina responsible for central vision. The hypothesis for the natural concentration is that lutein helps keep the eyes safe from oxidative stress and the high-energy photons of blue light. Various research studies have shown that a direct relationship exists between lutein intake and pigmentation in the eye.[1][2][3][4][5][6][7]
Lutein may play a role in Haidinger's brush, an entoptic phenomenon that allows humans to detect polarized light.
Dexeneración macular
Several studies show that an increase in macula pigmentation decreases the risk for eye diseases such as age-related macular degeneration (AMD).[8][9][10] The only randomized clinical trial to demonstrate a benefit for lutein in macular degeneration was a small study, in which the authors concluded that visual function is improved with lutein alone or lutein together with other nutrients and also that more study was needed.[9]
There is epidemiological evidence of a relationship between low plasma concentrations of lutein and zeaxanthin, and an increased risk of developing age-related macular degeneration (AMD). Some studies support the view that supplemental lutein and/or zeaxanthin help protect against AMD.[11]
In 2007, in a six-year study, John Paul SanGiovanni of the National Eye Institute, Maryland found that lutein and zeaxanthin (nutrients in eggs, spinach and other green vegetables) protect against blindness (macular degeneration), affecting 1.2 million Americans, mostly after age 65. Lutein and zeaxanthin reduce the risk of AMD.[11]
In 2013, findings of the Age-related Eye Disease Study 2 were reported in JAMA; AREDS2 was a five-year study designed to test whether the original AREDS formulation that was shown to reduce progression of age-related macular degeneration by 25 percent would be improved by adding omega-3 fatty acids; adding lutein and zeaxanthin; removing beta-carotene; or reducing zinc.[12] In AREDS2, participants took one of four AREDS formulations: the original AREDS formulation, AREDS formulation with no beta-carotene, AREDS with low zinc, AREDS with no beta-carotene and low zinc.[12] In addition, they took one of four additional supplement or combinations including lutein and zeaxanthin (10 mg and 2 mg), omega-3 fatty acids (1,000 mg), lutein/zeaxanthin and omega-3 fatty acids, or placebo.[12] The study reported that there was no overall additional benefit from adding omega-3 fatty acids or lutein and zeaxanthin to the formulation.[12] However, the study did find benefits in two subgroups of participants: those not given beta-carotene, and those who had very little lutein and zeaxanthin in their diets.[12] Removing beta-carotene did not curb the formulation's protective effect against developing advanced AMD, which was important given that high doses of beta-carotene had been linked to higher risk of lung cancers in smokers.[12] It was recommended to replace beta-carotene with lutein and zeaxanthin in future formulations for these reasons.[12]
Cataratas
There is also epidemiological evidence that increasing lutein and zeaxanthin intake lowers the risk of cataract development.[11][13] Consumption of more than 2.4 mg of lutein/zeaxanthin daily from foods and supplements was significantly correlated with reduced incidence of nuclear lens opacities, as revealed from data collected during a 13- to 15-year period in the Nutrition and Vision Project (NVP).[14]
Fotofobia
A study by Stringham and Hammond, published in the January/February 2010 issue of Journal of Food Science, discusses the improvement in visual performance and decrease in light sensitivity (glare) in subjects taking 10 mg lutein and 2 mg zeaxanthin per day.[15]
En nutrición
Lutein is a natural part of human diet when fruits and vegetables are consumed. For individuals lacking sufficient lutein intake, lutein-fortified foods are available, or in the case of elderly people with a poorly absorbing digestive system, a sublingual spray is available. As early as 1996, lutein has been incorporated into dietary supplements. While no recommended daily allowance currently exists for lutein as for other nutrients, positive effects have been seen at dietary intake levels of 6–10 mg/day.[16] The only definitive side effect of excess lutein consumption is bronzing of the skin (carotenodermia).
The functional difference between lutein (free form) and lutein esters is not entirely known. It is suggested that the bioavailability is lower for lutein esters, but much debate continues.[17]
As a food additive, lutein has the E number E161b (INS number 161b) and is extracted from the petals of marigold (Tagetes erecta).[18] It is approved for use in the EU[19] and Australia and New Zealand[20] but is banned in the USA.[Cómpre referencia]
Some foods are considered good sources of the nutrients:[11][21][22][23]
| Product | Lutein/zeaxanthin (micrograms per hundred grams) |
|---|---|
| nasturtium (yellow flowers, lutein levels only) | 45,000 |
| kale (raw) | 39,550 |
| kale (cooked) | 18,246 |
| dandelion leaves (raw) | 13,610 |
| nasturtium (leaves, lutein levels only) | 13,600 |
| turnip greens (raw) | 12,825 |
| spinach (raw) | 12,198 |
| spinach (cooked) | 11,308 |
| swiss chard (raw or cooked) | 11,000 |
| turnip greens (cooked) | 8440 |
| collard greens (cooked) | 7694 |
| watercress (raw) | 5767 |
| garden peas (raw) | 2593 |
| romaine lettuce | 2312 |
| zucchini | 2125 |
| brussels sprouts | 1590 |
| pistachio nuts | 1205 |
| broccoli | 1121 |
| carrot (cooked) | 687 |
| Maize/corn | 642 |
| egg (hard boiled) | 353 |
| avocado (raw) | 271 |
| carrot (raw) | 256 |
| kiwifruit | 122 |
Valor comercial
The lutein market is segmented into pharmaceutical, nutraceutical, food, pet foods, and animal and fish feed.
- The pharmaceutical market is estimated to be about US$190 million, nutraceutical and food is estimated to be about US$110 million.
- Pet foods and other applications are estimated at US$175 million annually.
Apart from the customary age-related macular degeneration applications, newer applications are emerging in cosmetics, skins and as an antioxidant. It is one of the fastest growing areas of the US$2 billion carotenoid market.[24]
Notas
- ↑ Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M (August 1980). "Diet-related macular anomalies in monkeys". Invest. Ophthalmol. Vis. Sci. 19 (8): 857–63. PMID 7409981.
- ↑ Johnson EJ, Hammond BR, Yeum KJ; et al. (June 2000). "Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density". Am. J. Clin. Nutr. 71 (6): 1555–62. PMID 10837298.
- ↑ Landrum, J., et al. Serum and macular pigment response to 2.4 mg dosage of lutein. in ARVO. 2000.
- ↑ Berendschot TT, Goldbohm RA, Klöpping WA, van de Kraats J, van Norel J, van Norren D (October 2000). "Influence of lutein supplementation on macular pigment, assessed with two objective techniques". Invest. Ophthalmol. Vis. Sci. 41 (11): 3322–6. PMID 11006220.
- ↑ Aleman TS, Duncan JL, Bieber ML; et al. (July 2001). "Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome". Invest. Ophthalmol. Vis. Sci. 42 (8): 1873–81. PMID 11431456.
- ↑ Duncan JL, Aleman TS, Gardner LM; et al. (March 2002). "Macular pigment and lutein supplementation in choroideremia". Exp. Eye Res. 74 (3): 371–81. PMID 12014918. doi:10.1006/exer.2001.1126.
- ↑ Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM (February 2005). "Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys". Invest. Ophthalmol. Vis. Sci. 46 (2): 692–702. PMID 15671301. doi:10.1167/iovs.02-1192.
- ↑ Richer S (January 1999). "ARMD—pilot (case series) environmental intervention data". J Am Optom Assoc 70 (1): 24–36. PMID 10457679.
- ↑ 9,0 9,1 Richer S, Stiles W, Statkute L; et al. (April 2004). "Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial)". Optometry 75 (4): 216–30. PMID 15117055.
- ↑ Age-Related Eye Disease Study Research Group (October 2001). "A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8". Arch. Ophthalmol. 119 (10): 1417–36. PMC 1462955. PMID 11594942. doi:10.1001/archopht.119.10.1417.
- ↑ 11,0 11,1 11,2 11,3 SanGiovanni JP, Chew EY, Clemons TE; et al. (September 2007). "The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22". Arch. Ophthalmol. 125 (9): 1225–32. PMID 17846363. doi:10.1001/archopht.125.9.1225.
- ↑ 12,0 12,1 12,2 12,3 12,4 12,5 12,6 http://www.nei.nih.gov/news/pressreleases/050513.asp
- ↑ "Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women's Health Initiative.".
- ↑ Barker Fm, 2nd (2010). "Dietary supplementation: effects on visual performance and occurrence of AMD and cataracts.". Current medical research and opinion 26 (8): 2011–23. PMID 20590393. doi:10.1185/03007995.2010.494549.
- ↑ Stringham, James M.; et al. (January–February 2010). "The Influence of Dietary Lutein and Zeaxanthin on Visual Performance". Journal of Food Science 75 (1): R24–R29. PMID 20492192. doi:10.1111/j.1750-3841.2009.01447.x. Consultado o January 15, 2013.
- ↑ Seddon JM, Ajani UA, Sperduto RD; et al. (November 1994). "Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group". JAMA 272 (18): 1413–20. PMID 7933422. doi:10.1001/jama.272.18.1413.
- ↑ Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M (2002). "Esterification does not impair lutein bioavailability in humans". J Nutr 132 (12): 3668–73. PMID 12468605.
- ↑ WHO/FAO Codex Alimentarius General Standard for Food Additives
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Consultado o 2011-10-27.
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Consultado o 2011-10-27.
- ↑ Reuters, Study finds spinach, eggs ward off cause of blindness
- ↑ USDA National Nutrient Database for Standard Reference, Release 23 (2010)
- ↑ Niizu, P.Y.; Delia B. Rodriguez-Amaya (2005). "Flowers and Leaves of Tropaeolum majus L. as Rich Sources of Lutein". Journal of Food Science 70 (9): S605–S609. ISSN 1750-3841. doi:10.1111/j.1365-2621.2005.tb08336.x.
- ↑ FOD025C The Global Market for Carotenoids, BCC Research
