Diacilglicérido: Diferenzas entre revisións
Sen resumo de edición |
Sen resumo de edición |
||
| Liña 3: | Liña 3: | ||
Un '''diacilglicérido''', tamén chamado '''diacilglicerol''' (DAG) ou '''diglicérido''', é un [[acilglicérido]] formado por unha molécula de [[glicerol]] (glicerina) [[éster|esterificada]] con dous [[ácido graxo|ácidos graxos]]. |
Un '''diacilglicérido''', tamén chamado '''diacilglicerol''' (DAG) ou '''diglicérido''', é un [[acilglicérido]] formado por unha molécula de [[glicerol]] (glicerina) [[éster|esterificada]] con dous [[ácido graxo|ácidos graxos]]. |
||
Os ácidos graxos están esterificados nos carbonos 1 e 2 ou no 1 e 3, polo que existen 1,2-diacilglicéridos e 1,3-diacilglicéridos. |
Os ácidos graxos están esterificados nos carbonos 1 e 2 ou no 1 e 3, polo que existen 1,2-diacilglicéridos e 1,3-diacilglicéridos <ref>IUPAC Goldbook [http://goldbook.iupac.org/G02647.html Glycerides]</ref>. |
||
== Uso como aditivo alimentario == |
== Uso como aditivo alimentario == |
||
Revisión como estaba o 18 de novembro de 2011 ás 17:16
Este artigo está a ser traducido ao galego por un usuario desta Wikipedia; por favor, non o edite. O usuario Miguelferig (conversa · contribucións) realizou a última edición na páxina hai 12 anos. Se o usuario non publica a tradución nun prazo de trinta días, procederase ó seu borrado rápido. |
Un diacilglicérido, tamén chamado diacilglicerol (DAG) ou diglicérido, é un acilglicérido formado por unha molécula de glicerol (glicerina) esterificada con dous ácidos graxos. Os ácidos graxos están esterificados nos carbonos 1 e 2 ou no 1 e 3, polo que existen 1,2-diacilglicéridos e 1,3-diacilglicéridos [1].
Uso como aditivo alimentario
Mono- and diacylglycerols are common food additives used to blend together certain ingredients, such as oil and water, which would not otherwise blend well.
The commercial source may be either animal (cow- or hog-derived) or vegetable, derived primarily from partially hydrogenated soy bean and canola oil. They may also be synthetically produced. They are often found in bakery products, beverages, ice cream, chewing gum, shortening, whipped toppings, margarine, and confections.
Funcións biolóxicas
Transdución de sinais

In biochemical signaling, diacylglycerol functions as a second messenger signaling lipid, and is a product of the hydrolysis of the phospholipid PIP2 (phosphatidyl inositol-bisphosphate) by the enzyme phospholipase C (PLC) (a membrane-bound enzyme) that, through the same reaction, produces inositol trisphosphate (IP3). Although inositol trisphosphate (IP3) diffuses into the cytosol, diacylglycerol (DAG) remains within the plasma membrane, due to its hydrophobic properties. IP3 stimulates the release of calcium ions from the smooth endoplasmic reticulum, whereas DAG is a physiological activator of protein kinase C (PKC). The production of DAG in the membrane facilitates translocation of PKC from the cytosol to the plasma membrane.
Diacylglycerol can be mimicked by the tumor-promoting compounds phorbol esters.
Outras
In addition to activating PKC, diacylglycerol has a number of other functions in the cell:
- a source for prostaglandins
- a precursor of the endocannabinoid 2-arachidonoylglycerol
- an activator of a subfamily of TRPC (Transient Receptor Potential Canonical) cation channels, TRPC3/6/7.
Metabolismo
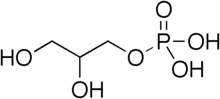
Synthesis of diacylglycerol begins with glycerol-3-phosphate, which is derived primarily from dihydroxyacetone phosphate, a product of glycolysis (usually in the cytoplasm of liver or adipose tissue cells). Glycerol-3-phosphate is first acylated with acyl-coenzyme A (acyl-CoA) to form lysophosphatidic acid, which is then acylated with another molecule of acyl-CoA to yield phosphatidic acid. Phosphatidic acid is then de-phosphorylated to form diacylglycerol.
Diacylglycerol is a precursor to triacylglycerol (triglyceride), which is formed in the addition of a third fatty acid to the diacylglycerol under the catalysis of diglyceride acyltransferase.
Since diacylglycerol is synthesized via phosphatidic acid, it will usually contain a saturated fatty acid at the C-1 position on the glycerol moiety and an unsaturated fatty acid at the C-2 position. [2]
Notas
- ↑ IUPAC Goldbook Glycerides
- ↑ Berg J, Tymoczko JL, Stryer L (2006). Biochemistry (6th ed. ed.). San Francisco: W. H. Freeman. ISBN 0716787245.
